Almased Clinical Studies: Liver and Renal Health
Background: Soy protein is used for meal replacement therapy in obesity, however the influence on renal function parameters is not adequately investigated. This study evaluates glomerular filtration rate (GFR) and renal plasma flow (RPF) in patients with the metabolic syndrome and healthy controls after ingestion of different amounts of soy protein.
Methods: 10 patients with the metabolic syndrome but no signs of kidney disease and 10 healthy controls ingested 1 g protein/kg body weight of a commercial soy-yoghurt-honeypreparation. The patient group was also given a protein challenge of 0.3 g/kg body weight.
Results: Baseline GFR and RPF both were significantly higher in the patient group (147 ± 34.8 vs. 116 ± 21.1 ml/min, p=0.01 and 848 ± 217 vs. 637 ± 121 ml/min, p=0.02) and were strongly correlated with body weight. Use of different algorithms to estimate GFR resulted in underestimation of GFR, particularly in the patients with the metabolic syndrome. The challenge with an acute protein load of 1g protein per kilogram body weight induced a significant increase in GFR and RPF in healthy controls (GFR: +12.6 ± 11.0 % (p=0.01), RPF: +13.6 ± 15.6 % (p=0.04)) and even more in patients with the metabolic syndrome (GFR: +31.5 ± 32.2 % (p=0.01); RPF: +19.4 ± 22.7 % (p=0.02)). The ingestion of 0.3 g protein/ kg body weight did not induce significant changes.
Conclusions: Basic renal function is changed in patients with the metabolic syndrome, even without microalbuminuria. In addition, there is an elevated susceptibility for protein load. However, the protein amount recommended for use in soy-protein based meal replacement therapy induced no significant changes.
Link to Study: Acute effect of a soy protein-rich meal-replacement application on renal parameters in patients with the metabolic syndrome
Download PDF: Acute effect of a soy protein-rich meal-replacement application on renal parameters
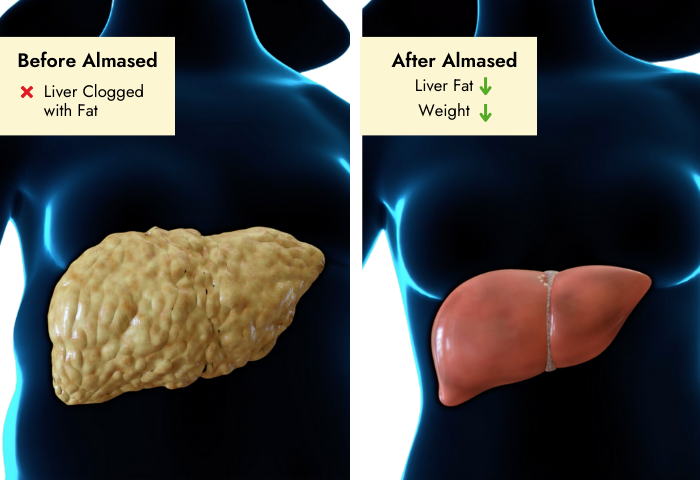
Background: Non-alcoholic steatohepatitis (NASH) has become one of the leading causes of liver disease in the western world. In obese patients weight reduction is recommended. Up to now there are no specific guidelines for weight loss in order to reduce hepatic fat content.
Aim: To investigate the effects of a 24-wk guided lifestyle intervention program compared to a meal replacement regimen based on soy protein.
Methods: Twenty-six subjects with NASH participated in a randomized single-center study. They were randomly assigned to either meal replacement group (MR-G) with soy-yogurt-honey preparation (Almased) or to guided lifestyle change group (LC-G) with endurance activity and nutrition counselling. Serum alanine transaminase (ALT), aspartate transaminase (AST), lipid parameters, and adipokines were measured. Liver fat content and lipid composition were determined by magnetic resonance imaging and magnetic resonance spectroscopy. Body fat mass and lean body mass were assessed using Bod Pod® device. Pre- and post-intervention monitoring of parameters was performed. Statistical analyses were conducted with SPSS software, results were expressed as median (interquartile range).
Results: Twenty-two subjects (MR-G, n = 11 and LC-G, n = 11) completed the study (9 women, 13 men; age 52.1 (15.0) years, body mass index (BMI) 32.3 (3.3) kg/m²). In both groups a significant weight loss was achieved (MR-G: -6.4 (3.6) kg, P < 0.01; LC-G: -9.1 (10.4) kg, P < 0.01). BMI dropped in both groups (MR-G: -2.3 (1.5) kg/m2, P = 0.003; LC-G: -3.0 (3.4) kg/m2, P = 0.006). Internal fat and hepatic lipid content were markedly reduced in both groups in comparable amount. There was a strong correlation between reduction in liver fat and decrease in ALT. Likewise, both groups showed an improvement in glycemic control and lipid profile. Changes in adipokines, particularly in adiponectin and leptin were closely related to intrahepatic lipid changes.
Conclusion: Comprehensive lifestyle intervention and meal replacement regimen have comparable effects on body and liver fat, as well as decrease in markers of hepatic inflammation among NASH patients.
Link to Study: Comprehensive lifestyle intervention vs Almased (soy protein-based meal regimen) in non-alcoholic steatohepatitis
Download PDF: Liver Study - Effect of Almased compared to Comprehensive Lifestyle
Almased Clinical Studies: Metabolism & Weight Loss
Background: High-protein diets and total diet replacements are becoming increasingly popular for weight loss; however, further research is needed to elucidate their impact on the mechanisms involved in weight regulation.
Objective: The aim of this inpatient metabolic balance study was to compare the impact of a high-protein total diet replacement (HP-TDR) versus a control diet (CON) on select components of energy metabolism in healthy adults of both sexes.
Methods: The acute intervention was a randomized, controlled, crossover design with participants allocated to 2 isocaloric arms: 1) HP-TDR: consisted of a soy-potein nutritional supplement (Almased) mixed with olive oil and low-fat milk (35% carbohydrate, 40% protein, and 25% fat) 2) CON: 55% carbohydrate, 15% protein, and 30% fat. Participants received the prescribed diets for 32 h while inside a whole-body calorimetry unit (WBCU). The first dietary intervention randomly offered in the WBCU was designed to maintain energy balance and the second matched what was offered during the first stay. Energy expenditure, macronutrient oxidation rates and balances, and metabolic blood markers were assessed. Body composition was measured at baseline using DXA.
Results: Forty-three healthy, normal-weight adults (19 females and 24 males) were included. Compared with the CON diet, the HP-TDR produced higher total energy expenditure [(EE) 81 ± 82 kcal/d, P <0.001], protein and fat oxidation rates (38 ± 34 g/d, P <0.001; 8 ± 20 g/d, P = 0.013, respectively), and a lower carbohydrate oxidation rate (-38 ± 43 g/d, P <0.001). Moreover, a HP-TDR led to decreased energy (-112 ± 85 kcal/d; P <0.001), fat (-22 ± 20 g/d; P <0.001), and carbohydrate balances (-69 ± 44 g/d; P <0.001), and increased protein balance (90 ± 32 g/d; P <0.001).
Conclusions: Our primary findings were that a HP-TDR led to higher total EE, increased fat oxidation, and negative fat balance. These results suggest that a HP-TDR may promote fat loss compared with a conventional isocaloric diet. These trials were registered at clinicaltrials.gov as NCT02811276 and NCT03565510.
Download PDF: Almased Study on How Almased Increases Calorie Burn and Leads to Fat Reduction
Aim: Our objective was to assess alterations in metabolic risk factors, body weight, fat mass and hormonal parameters following 6 weeks of lifestyle intervention with increased physical activity and either a meal-replacement regimen or a low calorie diet.
Research methods and procedures: 90 overweight or obese subjects (age 47 +/- 7.5 years, weight 90.6 +/- 11.3 kg, BMI 31.5 +/- 2.3) were included in this randomized controlled clinical trial. Subjects in the fat-restricted low-calorie-diet group (LCD-G; n = 30) received 2 dietary counseling sessions and instructions on how to increase physical activity. Subjects in the meal-replacement-diet group (MRD-G; n = 60) received the same lifestyle education and were instructed to replace 2 daily meals by a low-calorie high soy-protein drink.
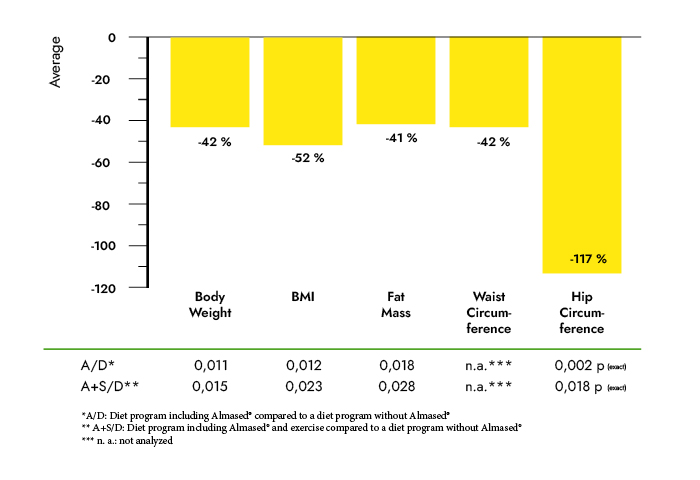
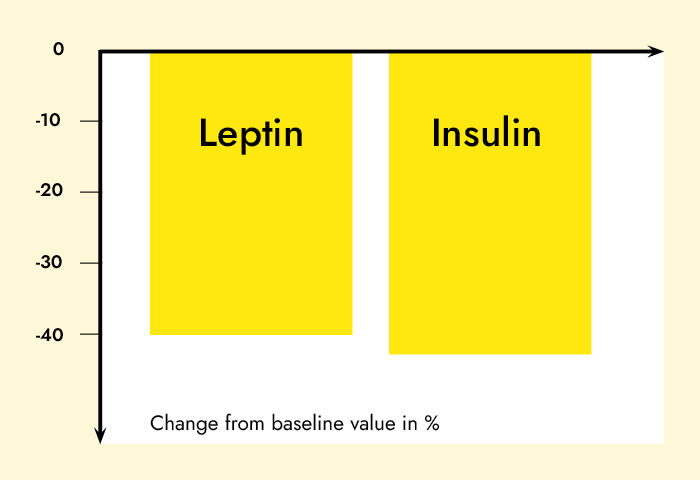
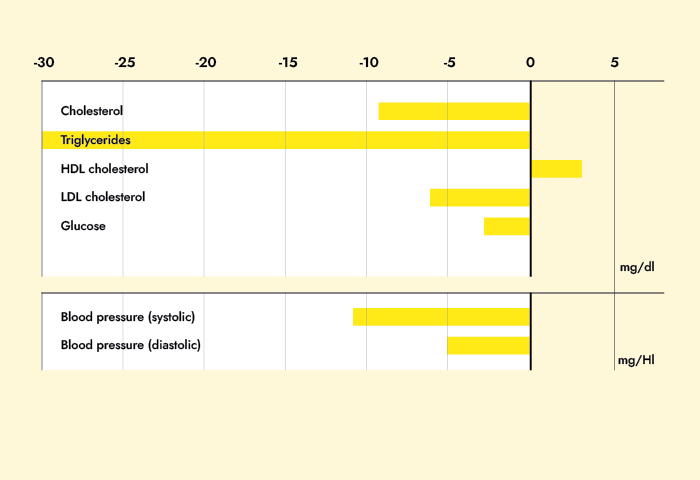
Results: Subjects in the MRD-G lost significantly more weight (6.4 vs. 3.1 kg, p < 0.01) and fat mass (5.1 vs. 2.8 kg, p < 0.01) than the LCD-G. Most metabolic risk parameters were reduced in both the MRD-G and the LCD-G; however, subjects in the MRD-G showed a higher reduction in waist circumference (6.1 vs. 1.8 cm, p < 0.01) and a larger decrease in triglycerides (-19.6 vs. +12.5 mg/dl, p < 0.01). The prevalence of the metabolic syndrome was reduced in subjects in the MRD-G only (-12%, p < 0.05) compared to an unchanged risk score in the LCD-G. The reductions in leptin (18.2 vs. 6.97 ng/ml) and insulin (4.92 vs. 0.58 microU/ml) were only significant in the MRD-G (p < 0.01).
Discussion: Our data suggest that even over a short period of time, a meal-replacement diet is more effective in reducing metabolic risk factors, insulin, and leptin, and in improving anthropometric measures than a fat-restricted low-calorie diet.
Link to Study: Effect of meal replacement on metabolic risk factors in overweight and obese subjects - PubMed (nih.gov)
Download PDF: Almased Study on How Almased Reduces Metabolic Risk Factors
HTML: Effect of meal replacement on metabolic risk factors in overweight and obese subjects
Objective: To determine the changes in endurance capacity as well as in metabolic, hormonal and inflammatory markers induced by endurance training combined with a soy,protein based supplement.
Design: Randomized controlled study consisting of moderate endurance training without (GO) or with (G1) a soy protein based supplement (Almased).
Subjects: Two groups of 15 subjects (10 males and 5 females in each group): healthy sports students aged 23.6 +/- 1.9 years.
Measurements: Body composition (body mass (BM), body density (BD) by air displacement) and physical fitness (determined by treadmill ergometry) were measured at baseline and after 6 weeks of the intervention; changes in circulating metabolic and hormonal parameters (glucose, lactate, urea, uric acid, ammonia, cortisol, insulin, IGF-1), and exercise-induced stress and inflammatory markers (CK, LDH, myoglobin, hs-CRP, IL-6, IL-10, blood cell counts) were determined after the intervention period in afield test (11.5 km running on hilly ground).
Results: 30 participants completed the 6-week study; 28 students were able to perform the field test. No significant changes in BM and BD were noted after intervention with only slight increases in running performance and maximum aerobic capacity in the total group (2%, p=0.016). Subjects in the G1 group showed significant improvements in running velocity and lower lactate values following the intervention (-12%, p=0,003). In addition, the G1 group showed significantly lower differences in the exercise-induced increase of metabolic parameters (triglycerides, uric acid) and insulin in the post-exercise recovery period.
Conclusions: Our data suggest that moderate endurance training in combination with a soy-based protein supplement improves aerobic energy supply and metabolic function in healthy sports students, even without changes in body composition and without changes in the exercise-induced stress and inflammatory reaction.
Link to Study: A soy-based supplement alters energy metabolism but not the exercise-induced stress response - PubMed (nih.gov)
Download PDF: Almased alters energy metabolism
HTML: A soy-based supplement alters energy metabolism but not the exercise-induced stress response
Background: The present study examines changes in body weight, fat mass, metabolic and hormonal parameters in overweight and obese pre- and postmenopausal women who participated in a weight loss intervention.
Methods: Seventy-two subjects were included in the analysis of this single arm study (premenopausal: 22 women, age 43.7 +/- 6.4 years, BMI 31.0 +/- 2.4 kg/m2; postmenopausal: 50 women, age 58.2 +/- 5.1 years, BMI 32.9 +/- 3.7 kg/m2). Weight reduction was achieved by the use of a meal replacement (Almased) and fat-reduced diet. In addition, from week 6 to 24 participants attended a guided exercise program. Body composition was analyzed with the Bod Pod(R). Blood pressures were taken at every visit and blood was collected at baseline and closeout of the study to evaluate lipids, insulin, cortisol and leptin levels.
Results: BMI, fat mass, waist circumference, systolic blood pressure, triglycerides, glucose, leptin and cortisol were higher in the postmenopausal women at baseline. Both groups achieved a substantial and comparable weight loss (pre- vs. postmenopausal: 6.7 +/- 4.9 vs 6.7 +/- 4.4 kg; n.s.). However, in contrast to premenopausal women, weight loss in postmenopausal women was exclusively due to a reduction of fat mass (-5.3 +/- 5.1 vs -6.6 +/- 4.1 kg; p < 0.01). In premenopausal women 21% of weight loss was attributed to a reduction in lean body mass. Blood pressure, triglycerides, HDL-cholesterol, and glucose improved significantly only in postmenopausal women whereas total cholesterol and LDL-cholesterol were lowered significantly in both groups.
Conclusion: Both groups showed comparable weight loss and in postmenopausal women weight loss was associated with a pronounced improvement in metabolic risk factors thereby reducing the prevalence of metabolic syndrome.
Download PDF: Almased study on effect of Almased on weight loss on measures and metabolisk risk factors in menopausal women
Introduction: The need to repeatedly emphasize the importance of obesity and its direct link to lifestyle arises from the fact that, despite all efforts, the proportion of overweight people in our population continues to increas despite all efforts [1, 2]. Obesity is more than just a cosmetic problem, as it is epidemiologically proven that overweight people are a risk group for atherosclerotic and metabolic diseases that require therapy [3, 4].
In turn, Germany is being overrun by a wave of weight reduction programs. Around 200 such programs are currently programs are currently offered by various institutions. Only a few programs offer reliable success, and only a few have been evaluated and meet the criteria of continuous quality management. Despite all this, there is no doubt that only a lasting change in dietary and activity behavior towards an energetically balanced lifestyle and a simultaneous improvement in dietary quality can lead to lasting success [5].
Against this background, the Department of Rehabilitative and Preventive Sports Medicine at the University Hospital of Freiburg has already in October 2003 [6] reported on the first successes of a controlled and randomized study on the reduction of increased body weight in adults. After the publication of the half-year results in the past, the one-year results should now show that the feasibility of weight and fat mass reduction according to the calorie balance approach is possible with reasonable effort for the participants, is not possible. The scientific and practical results obtained in this way should also serve as a basis for the creation of a standardized training program for the treatment of obesity and its associated risk factors [7].
Link to Study: Weight reduction by lifestyle intervention
Download PDF (GER): Weight reduction by lifestyle intervention
Download PDF (ENG): Weight reduction by lifestyle intervention
Abstract: The changed living conditions and associated behaviors in the Western world have in many cases overridden previously successful basic biological principles. The consequence, which is visible to everyone and a source of suffering for many, is a loss of health literacy and responsibility, clearly recognizable in the dramatic increase in obesity and its secondary diseases [22, 24]. There appears to be only one comprehensive and effective solution to the problem of obesity: A permanent change in dietary and activity behavior towards an energetically balanced lifestyle while improving diet quality. Against this background, various intervention approaches for weight reduction were compared in terms of their effectiveness in a controlled, randomized study. The aim of the study was, on the one hand, to demonstrate the feasibility of weight and fat mass reduction using a model approach to energy balance. On the other hand, scientific and practical experience in the care of overweight adults was to be gathered in order to develop a standardized intervention program for the treatment of obesity and its associated risk factors.
Link to Study: Weight reduction is feasible
Download PDF (GER): Weight reduction is feasible
Download PDF (ENG): Weight reduction is feasible
Abstract: Although meal replacement can lead to weight reduction, there is uncertainty whether this dietary approach implemented into a lifestyle programme can improve long-term dietary intake. In this subanalysis of the Almased Concept against Overweight and Obesity and Related Health Risk (ACOORH) study (n = 463), participants with metabolic risk factors were randomly assigned to either a meal replacement-based lifestyle intervention group (INT) or a lifestyle intervention control group (CON). This subanalysis relies only on data of participants (n = 119) who returned correctly completed dietary records at baseline, and after 12 and 52 weeks. Both groups were not matched for nutrient composition at baseline. These data were further stratified by sex and also associated with weight change. INT showed a higher increase in protein intake related to the daily energy intake after 12 weeks (+6.37% [4.69; 8.04] vs. +2.48% [0.73; 4.23], p < 0.001) of intervention compared to CON. Fat and carbohydrate intake related to the daily energy intake were more strongly reduced in the INT compared to CON (both p < 0.01). After sex stratification, particularly INT-women increased their total protein intake after 12 (INT: +12.7 g vs. CON: −5.1 g, p = 0.021) and 52 weeks (INT: +5.7 g vs. CON: −16.4 g, p = 0.002) compared to CON. Protein intake was negatively associated with weight change (r = −0.421; p < 0.001) after 12 weeks. The results indicate that a protein-rich dietary strategy with a meal replacement can improve long-term nutritional intake, and was associated with weight loss.
Download PDF: Effects of a protein-rich, low-glycaemic meal replacement on changes in dietary intake and body weight
Abstract: Numerous studies have found that increased body size (weight or body mass index) is a risk factor for breast cancer development, recurrence, and death. The detrimental relationship between body size and breast cancer recurrence may be more pronounced among women with estrogen receptor (ER)/progesterone receptor (PR)-negative breast cancer. Considering the limited availability of treatments, and the association between body size and recurrence, alternative treatments are needed for ER/PR-negative breast cancer survivors, particularly overweight survivors. The objective of this pilot study was to examine the feasibility of a 12-week, multi-component meal-replacement weight loss intervention among overweight or obese ER/PR-negative breast cancer survivors; and to obtain preliminary data on changes in anthropometrics, biomarkers, and health-related quality of life (QOL). The 12-week intervention included a portion-controlled diet (including meal replacements) and a multi-component intervention (including behavioral techniques, diet modification, physical activity, and social support). The goal of the intervention was to help participants lose 5% or more of their initial weight by reducing their caloric intake and increasing their physical activity (to at least 15 minutes each day). Paired t-tests assessed changes in continuous measures. Body weight was measured weekly and mixed-model regression analysis assessed change in weight over time. Nineteen ER/PR-negative breast cancer survivors with a mean age of 59 years participated in the study. All but two of the participants completed the 12-week intervention. Women lost an average of 6.3 ± 4.9 kg (P < 0.001), equivalent to 7.5% of their baseline weight. There were significant reductions in waist circumference (P = 0.001), percent fat mass (P < 0.001), total cholesterol (P = 0.026), and triglycerides (P = 0.002); and improvements in health-related QOL (P = 0.017). Findings suggested that a meal-replacement weight loss approach among ER/PR-negative breast cancer survivors was feasible and was well received.
Link to Study: Weight Loss Intervention in Survivors of ER/PR-negative Breast Cancer
Download PDF: Weight Loss Intervention in Survivors of ER/PR-negative Breast Cancer
HTML: Weight Loss Intervention in Survivors of ER/PR-negative Breast Cancer
Almased Clinical Studies: Hunger & Appetite Regulation
Background: The aim of this study was to compare the impact of a high-protein meal replacement (HP-MR) versus a control (CON) breakfast on exercise metabolism.
Methods: In this acute, randomized controlled, cross-over study, participants were allocated into two isocaloric arms: (a) HP-MR: 30% carbohydrate, 43% protein, and 27% fat; (b) CON: 55% carbohydrate, 15% protein, and 30% fat. Following breakfast, participants performed a moderate-intensity aerobic exercise while inside a whole-body calorimetry unit. Energy expenditure, macronutrient oxidation, appetite sensations, and metabolic blood markers were assessed.
Results: Forty-three healthy, normal-weight adults (24 males) participated. Compared to the CON breakfast, the HP-MR produced higher fat oxidation (1.07 ± 0.33 g/session; p = 0.003) and lower carbohydrate oxidation (-2.32 ± 0.98 g/session; p = 0.023) and respiratory exchange ratio (-0.01 ± 0.00; p = 0.003) during exercise. After exercise, increases in hunger were lower during the HP-MR condition. Changes in blood markers from the fasting state to post-exercise during the HP-MR condition were greater for insulin, peptide tyrosine-tyrosine, and glucagon-like peptide 1, and lower for low-density lipoprotein cholesterol, triglyceride, and glycerol.
Conclusions: Our primary findings were that an HP-MR produced higher fat oxidation during the exercise session, suppression of hunger, and improved metabolic profile after it.
Purpose: Dietary intake can affect energy homeostasis and influence body weight control. The aim of this study was to compare the impact of high-protein total diet replacement (HP-TDR) versus a control (CON) diet in the regulation of food intake and energy homeostasis in healthy, normal-weight adults.
Methods: In this acute randomized controlled, cross-over study, participants completed two isocaloric arms: a) HP-TDR: 35% carbohydrate, 40% protein, and 25% fat; b) CON: 55% carbohydrate, 15% protein, and 30% fat. The diets were provided for 32 h while inside a whole-body calorimetry unit. Appetite sensations, appetite-related hormones, and energy metabolism were assessed.
Results: Forty-three healthy, normal-weight adults (19 females) participated. Appetite sensations did not differ between diets (all p > 0.05). Compared to the CON diet, the change in fasting blood markers during the HP-TDR intervention was smaller for peptide tyrosine-tyrosine (PYY; - 18.9 ± 7.9 pg/mL, p = 0.02) and greater for leptin (1859 ± 652 pg/mL, p = 0.007). Moreover, postprandial levels of glucagon-like peptide 1 (1.62 ± 0.36 pM, p < 0.001) and PYY (31.37 ± 8.05 pg/mL, p < 0.001) were higher in the HP-TDR. Significant correlations were observed between energy balance and satiety (r = - 0.41, p = 0.007), and energy balance and PFC (r = 0.33, p = 0.033) in the HP-TDR.
Conclusion: Compared to the CON diet, the HP-TDR increased blood levels of anorexigenic hormones. Moreover, females and males responded differently to the intervention in terms of appetite sensations and appetite-related hormones.
Download PDF: Almased meal replacement diet alters regulation of food intake and energy balance
Purpose: Lifestyle interventions including meal replacement are suitable for prevention and treatment of obesity and type-2-diabetes.
Methods: Since leptin is involved in weight regulation, we hypothesised that a meal replacement-based lifestyle intervention would reduce leptin levels more effectively than lifestyle intervention alone. In the international, multicentre, randomised-controlled ACOORH-trial (Almased-Concept-against-Overweight-and-Obesity-and-Related- Health-Risk), overweight or obese participants with metabolic syndrome criteria (n = 463) were randomised into two groups and received telemonitoring devices and nutritional advice. The intervention group additionally used a protein-rich, low-glycaemic meal replacement. Data were collected at baseline, after 1, 3, 6, and 12 months. All datasets providing leptin data (n = 427) were included in this predefined subanalysis.
Results: Serum leptin levels significantly correlated with sex, body mass index, weight, and fat mass at baseline (p < 0.0001). Stronger leptin reduction has been observed in the intervention compared to the control group with the lowest levels after 1 month of intervention (estimated treatment difference −3.4 µg/L [1.4; 5.4] for females; −2.2 µg/L [1.2; 3.3] for males; p < 0.001 each) and was predictive for stronger reduction of body weight and fat mass (p < 0.001 each) over 12 months. Strongest weight loss was observed after 6 months (−5.9 ± 5.1 kg in females of the intervention group vs. −2.9 ± 4.9 kg in the control group (p < 0.0001); −6.8 ± 5.3 kg vs. −4.1 ± 4.4 kg (p = 0.003) in males) and in those participants with combined leptin and insulin decrease.
Conclusion: A meal replacement-based lifestyle intervention effectively reduces leptin which is predictive for long-term weight loss.
Download PDF: Almased Study -Leptin Reduction Predictive for Long-Term Weight Loss
Objective: The present study investigated the postprandial glycemic and insulinemic responses, the levels of satiety-related proteins, and substrate use after a single dose of a meal replacement (MR) with a high soy protein content and a low glycemic index (GI). The results were compared with a standardized breakfast showing a high GI and a low protein content.
Methods: Eleven overweight or obese male subjects with the metabolic syndrome and insulin resistance were included in the study. In the morning, each subject consumed, in a randomized design, 65 g of a MR or an isocaloric standardized breakfast. Four hours after breakfast, all subjects consumed the same standardized lunch. Blood levels of glucose, insulin, ghrelin, protein YY(PYY), oxygen uptake, and carbon dioxide production were determined and the respiratory quotient and substrate use were calculated.
Results: The glycemic and insulinemic responses were considerably higher after the standardized breakfast. In addition, in these obese insulin-resistant subjects, the postprandial decease in fat oxidation was significantly less pronounced after intake of the MR. This effect was also detectable after lunch in terms of a second meal effect. Ghrelin levels were significantly lower 2 h after the intake of the MR and PYY levels tended higher.
Conclusion: Compared with the high GI/low-protein SB, a high soy protein MR with a low GI was associated with lower glycemia and insulinemia and relatively higher fat oxidation in the postprandial period. Together with a favorable course of appetite-regulating hormones, this could further help to explain the beneficial role of MR regimines high in soy protein for weight reduction and improvement of metabolic risk factors.
Link to Study: Fuel selection and appetite-regulating hormones after intake of a soy protein-based meal replacement - PubMed (nih.gov)
Download PDF: Almased meal replacement shakes effect on fuel selection and appetite hormones
HTML: Fuel selection and appetite-regulating hormones after intake of a soy protein-based meal replacement
Background and Purpose: The increasing prevalence of overweight and obese adults warrants improved dietary strategies for weight management and metabolic control. Hence, the objective of this study was to investigate the effects of a high-protein diet on leptin regulation.
Methods: This study was a secondary analysis of data collected from a randomized controlled trial, conducted in 90 overweight adults (age: 47.5 ± 7.5 yrs.; BMI: 31.5 ± 2.3 kg/m2) who were followed over a 24-week control period. Changes in leptin levels were quantified to determine the influence of age, gender, leptin baseline levels, weight loss and intervention type. Participants were randomized into 3 interventions groups: 1) therapeutic lifestyle changes, (LS); 2) standardized meal replacement (Almased®) (MR); and 3) standardized meal replacement accompanied by supervised physical training (MRPT). For the analyses, both diet groups (MR, MRPT) were pooled into one common group and compared to the LS group in a parallel two-group study design with endpoint assessment after 24 weeks of intervention.
Results: In total, 83 participants completed the 24-week study. Significant improvements in body composition and metabolic regulation occurred in all intervention participants regardless of their group assignment (LS; MR, MRPT). Participants' consumption of the meal replacement (MR; MRPT) had an independent, significant effect on serum leptin levels (-15.5 ± 7.5 and -12.5 ± 7.8 vs. -8.7 ± 6.1 ng/ml). Greater body weight reductions were also observed in the diet groups (-8.9 ± 3.9 kg) compared to the LS group (-6.2 ± 4.2 kg). Conclusions our findings suggest that meal replacement can safely and effectively produce significant weight loss, which may be in part due to a reduction in plasma leptin levels. ClinicalTrials.gov Identifier: NCT00356785.
Link to Study: The influence of a meal replacement formula on leptin regulation in obese adults
Download PDF: The influence of a meal replacement formula on leptin regulation in obese adults
HTML: The influence of a meal replacement formula on leptin regulation in obese adults
Almased Clinical Studies: Healthy Blood Sugar Control
Objective: The aim of this study was to investigate the effect of a 6-wk intervention with either lifestyle intervention (increased physical activity and a low-calorie diet) or a meal replacement regimen on glycemic control in patients who are prediabetic and have impaired fasting glucose.
Methods: Forty-two overweight or obese men and women (age 54 ± 8 y; weight 95.1 ± 11.9 kg; body mass index [BMI] 32.8 ± 2.89 kg/m(2)) were included in this randomized controlled clinical trial. Patients in the lifestyle group (LS; n = 14) received dietary counseling sessions (fat-restricted low-calorie diet) and instructions on how to increase physical activity. Patients in the meal replacement group (MR; n = 28) were instructed to replace two daily meals with Almased, a low-calorie, high soy-protein drink with a low glycemic index.
Results: Both interventions resulted in a significant decrease in body weight and BMI, although the reduction was more pronounced (P < 0.05) in the MR group. In both groups, glucose concentrations decreased significantly (LS: -12 mg/dL, P < 0.01; MR: -11 mg/dL, P < 0.01), and mean glucose levels returned to the normal range. Insulin (LS: -1 μU/mg [not significant]; MR: -6.3 μU/mg, P < 0.01) and homeostasis model assessment of insulin resistance (HOMA-IR; LS -0.92, P < 0.01; MR: -2.1, P < 0.01) were also significantly lower following both interventions; again improvements were more pronounced in the MR group (insulin: P < 0.05; HOMA P < 0.01) CONCLUSION: It can be concluded that meal replacement is an effective intervention for rapid improvement of elevated fasting glucose and increased insulin concentrations, these being important biomarkers of the prediabetic state. The 6-wk intervention has shown that the effect of meal replacement on fasting blood glucose was comparable to the effect of lifestyle intervention. The alterations in BMI, insulin, and HOMA-IR were significantly more pronounced following the meal replacement regimen.
Download PDF: Almased improves blood glucose levels
Objective: Lifestyle interventions are the foundation of treatment in newly diagnosed type 2 diabetes. However, their therapeutic potential in advanced disease stages is unknown. We evaluated the efficacy of the Telemedical Lifestyle intervention Program (TeLiPro) in improving metabolic control in advanced-stage type 2 diabetes.
Research design and methods: In this single-blind, active comparator, intervention study, patients with type 2 diabetes (with glycated hemoglobin [HbA1c] ≥7.5% [58.5 mmol/mol]), and BMI ≥27 kg/m2 and on ≥2 antidiabetes medications) were recruited in Germany and randomized 1:1 using an electronically generated random list and sealed envelopes into two parallel groups. The data analyst was blinded after assignment. The control group (n = 100) got weighing scales and step counters and remained in routine care. The TeLiPro group (n = 102) additionally received telemedical coaching including medical-mental motivation, Almased - a formula diet, and self-monitored blood glucose for 12 weeks. The primary end point was the estimated treatment difference in HbA1c reduction after 12 weeks. All available values per patient (n = 202) were analyzed. Analyses were also performed at 26 and 52 weeks of follow-up.
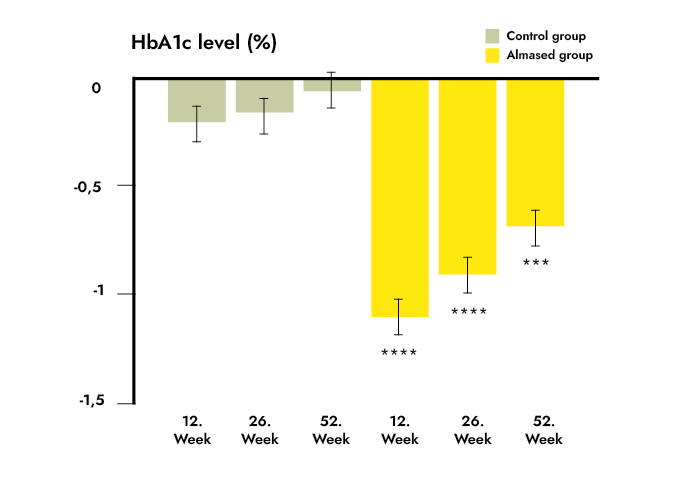
Results: HbA1c reduction was significantly higher in the TeLiPro group (mean ± SD -1.1 ± 1.2% vs. -0.2 ± 0.8%; P < 0.0001). The estimated treatment difference in the fully adjusted model was 0.8% (95% CI 1.1; 0.5) (P < 0.0001). Treatment superiority of TeLiPro was maintained during follow-up (week 26: 0.6% [95% CI 1.0; 0.3], P = 0.0001; week 52: 0.6% [0.9; 0.2], P < 0.001). The same applies for secondary outcomes: weight (TeLiPro -6.2 ± 4.6 kg vs. control -1.0 ± 3.4 kg), BMI (-2.1 ± 1.5 kg/m2 vs. -0.3 ± 1.1 kg/m2), systolic blood pressure (-5.7 ± 15.3 mmHg vs. -1.6 ± 13.8 mmHg), 10-year cardiovascular disease risk, antidiabetes medication, and quality of life and eating behavior (P < 0.01 for all). The effects were maintained long-term. No adverse events were reported.
Conclusions: In advanced-stage type 2 diabetes, TeLiPro can improve glycemic control and may offer new options to avoid pharmacological intensification.
Download PDF: Telemedical Lifestyle Intervention Program using Almased in Advanced Stages of Type 2 Diabetes
Background: Formula diets can improve glycemic control or can even induce remission in type 2 diabetes. We hypothesized that especially an individualized intense meal replacement by a low-carbohydrate formula diet with accompanied self-monitoring of blood glucose (SMBG) contributes to long-term improvements in HbA1c, weight, and cardiometabolic risk factors in poorly controlled type 2 diabetes.
Methods: Type 2 diabetes patients were randomized into either a moderate group (M-group) with two Almased meal replacements/day (n = 160) or a stringent group (S-group) with three Almased meal replacements/day (n = 149) during the first week of intervention (1300⁻1500 kcal/day). Subsequently, both groups reintroduced a low-carbohydrate lunch based on individual adaption due to SMBG in weeks 2⁻4. After week 4, breakfast was reintroduced until week 12. During the follow-up period, all of the participants were asked to continue replacing one meal per day until the 52-weeks follow-up. Additionally, an observational control group (n = 100) remained in routine care. Parameters were compared at baseline, after 12 and 52 weeks within and between all of the groups.
Results: 321 participants (83%) completed the acute meal replacement phase after 12 weeks and 279 participants (72%) the whole intervention after 52 weeks. Both intervention groups achieved improvements in HbA1c, fasting blood glucose, blood pressure, and weight (all p < 0.001) within 12 weeks. However, these results were not significantly different between both of the intervention groups. The estimated treatment difference in HbA1c reduction was (mean (95% confidence interval [CI]) -0.10% with 95% CI [-0.40; 0.21] also (p > 0.05) (S-group vs. M-group) not statistically different after 12 weeks. However, only the S-group showed a clinically relevant improvement in HbA1c of -0.81% [-1.06; -0.55] (p < 0.001) after 52 weeks of follow-up, whereas HbA1c was not statistically different between the M- and control group.
Conclusion: Individualized meal replacement with SMBG demonstrated beneficial effects on HbA1c and cardiometabolic parameters in type 2 diabetes. Furthermore, the initiation of a weight loss program with one week of full meal replacement (three meals per day) resulted in a clinically relevant long-term HbA1c reduction, as compared to an observational control group that had standard care.
Download PDF: Almased Meal Replacement Therapy Improves Glycemic Control
Background: Lifestyle interventions have been shown to reverse hyperglycemia to normoglycemia. However, these effects are not long-lasting and are accompanied with high dropout rates. As formula diets have been shown to be simple in usage and effective in improving glycemic control, we hypothesised that adding a low-carbohydrate and energy deficit formula diet to a low-intensity lifestyle intervention is superior in reversing prediabetes compared with lifestyle intervention alone.
Methods & Results: In this predefined subanalysis of an international, multicenter randomised controlled trial (Almased Concept against Overweight and Obesity and Related Health Risk (ACOORH) study (ID DRKS00006811)), 141 persons with prediabetes were randomised (1:2) into either a control group with lifestyle intervention only (CON, n = 45) or a lifestyle intervention group accompanied with a formula diet (INT, n = 96). Both groups were equipped with telemonitoring devices. INT received, Almased, a low-carbohydrate formula diet substituting three meals/day (~1200 kcal/day) within the first week, two meals/day during week 2-4, and one meal/day during week 5-26 (1300-1500 kcal/day). Follow-up was performed after 52 weeks and 105 participants (75%, INT: n = 74; CON: n = 31) finished the 26-week intervention phase. Follow-up data after 52 weeks were available from 93 participants (66%, INT: n = 65; CON: n = 28). Compared with CON, significantly more INT participants converted to normoglycemia after 52 weeks (50% vs. 31%; p < 0.05). The risk reduction led to a number-needed-to-treat of 5.3 for INT.
Conclusion: Lifestyle intervention with a low-carbohydrate formula diet reduces prediabetes prevalence stronger than lifestyle intervention alone and is effective for type 2 diabetes prevention.
Download PDF: Prediabetes Convension to Normoglycemia
Background: Lifestyle interventions, including meal replacement, are effective in the prevention and treatment of type-2-diabetes and obesity.
Methods & Results: Since insulin is the key weight regulator, we hypothesised that the addition of meal replacement to a lifestyle intervention reduces insulin levels more effectively than lifestyle intervention alone. In the international multicentre randomised controlled ACOORH (Almased Concept against Overweight and Obesity and Related Health Risk) trial, overweight or obese persons who meet the criteria for metabolic syndrome (n = 463) were randomised into two groups. Both groups received nutritional advice focusing on carbohydrate restriction and the use of telemonitoring devices. The intervention group substituted all three main meals per day in week 1, two meals per day in weeks 2-4, and one meal per day in weeks 5-26 with, Almased, a protein-rich, low-glycaemic meal replacement. Data were collected at baseline and after 1, 3, 6 and 12 months. All datasets providing insulin data (n = 446) were included in this predefined subanalysis. Significantly higher reductions in insulin (-3.3 ± 8.7 µU/mL vs. -1.6 ± 9.8 µU/mL), weight (-6.1 ± 5.2 kg vs. -3.2 ± 4.6 kg), and inflammation markers were observed in the intervention group. Insulin reduction correlated with weight reduction and the highest amount of weight loss (-7.6 ± 4.9 kg) was observed in those participants with an insulin decrease > 2 µU/mL.
Conclusion: These results underline the potential for meal replacement-based lifestyle interventions in diabetes prevention, and measurement of insulin levels may serve as an indicator for adherence to carbohydrate restriction.
Download PDF: 12-Month Study - Almased Decreases Fasting Insulin and Inflammation Markers
Background and purpose: The increasing prevalence of overweight and obesity among adults, demands improved dietary strategies for weight management and metabolic competence. Hence, the objective of this study was to assess the short-term effects of breakfasts with varying macronutrient composition on blood glucose regulation, energy metabolism and satiety.
Methods: This study examined ten healthy males (25.6 ± 4.4 yrs; BMI 23.2 ± 0.9 kg/m2) fed isoenergetic breakfasts rich in either Carbohydrate [CH] (68% of energy), Fat [Fat] (64% of energy) or Protein [P] (35% of energy) or a breakfast which reflected the individuals Normal [N] breakfast composition. Blood glucose and lactate, resting oxygen consumption (VO2), Respiratory Quotient (RQ) and satiety feeling were measured. All breakfasts with the exception of the individual normal breakfast variant were isoenergetic and all contained the same amount of dietary fiber. As a non-dietary control, subjects drank 200 ml water on one test day, with the same metabolic parameters measured.
Results: Compared with the water control day, there was a significant macronutrient-induced change in the metabolic parameters. The most significant increases in blood glucose were found after the Carbohydrate breakfast and the individual normal breakfast, whereas the Fat and Protein-rich breakfasts induced comparatively smaller blood glucose responses. Only the Proteinrich breakfast led to significant increases in resting VO2 (up to 30%) without changes in RQ. Finally, the Protein-rich breakfast induced the highest satiety feeling.
Conclusions: Although the Protein-induced effects may initially appear minor, the combination of a reduced glycemic response, increased VO2, a proportionately high fat oxidation and a stronger satiety effect may support the use of this dietary approach for healthy weight management in normal weight men.
Link to Study: Effect of different isoenergetic breakfast compositions on blood glucosallocation and satiety
Download PDF: Effect of different isoenergetic breakfast compositions on blood glucosallocation and satiety
HTML: Effect of different isoenergetic breakfast compositions on blood glucosallocation and satiety
Summary: Several studies have suggested that the metabolic syndrome may alter the risk of developing a variety of cancers – colon, pancreas, breast, liver, gall bladder – and the associations are biologically plausible. The American key societies – Cancer Society, Diabetes and Heart Associations – have stated that the current approaches to health promotion and prevention of cardiovascular disease, cancer and diabetes do not approach the potential of existing knowledge. A concerted effort to increase application of public health and clinical interventions of known efficacy could substantially reduce the human and economic costs of these diseases. Moreover, cancer survivors received less care for other medical conditions. In addition to a scientifically trend-setting weight reduction study [7], we introduce here data of a dietary intervention study in non-insulin dependent diabetes mellitus (NIDDM), showing that a regular support for 26 weeks of the day-to-day eating habit with a high-soy-protein/milk/yoghurt formulation (Almased®, Bienenbuttel, Germany, St. Petersburg, FL, USA) down-regulates hormone (insulin, IL-6) and biochemical (fasting glucose, trigliceride levels) parameters which may contribute to the carcinogenic process.
Download PDF: Type 2 Diabetes Mellitus and Cancer – a Nutrition-oriented Intervention Study
HTML: Type 2 Diabetes Mellitus and Cancer – a Nutrition-oriented Intervention Study
Background: Despite high insulin doses, good glycaemic control is often lacking in type 2 diabetes patients and new therapeutic options are needed.
Methods: In a proof of principle study, an energy-restricted, protein-rich meal replacement (PRMR) was examined as a means of reducing insulin requirement, HbA1C and body weight. Obese type 2 diabetes patients (n = 22) with >100 U insulin per day replaced, in week 1, the three main meals with 50 g of PRMR (Almased-Vitalkost) each (= 4903 kJ day(-1) ). In weeks 2-4, breakfast and dinner were replaced, and, in weeks 5-12, only dinner was replaced. Clinical parameters were determined at baseline, and after 4, 8 and 12 weeks, as well as after 1.5 years of follow-up. The Wilcoxon signed-rank test was used for the intention-to-treat analysis and the Mann-Whitney U-test for subgroup analyses.
Results: The 12-week-programme was completed by 15 participants (68%). After 1 week, the mean insulin dose was reduced from 147 (75) U to 91 (55) U day(-1) (P = 0.0001), and to 65 (32) U (P < 0.0001) after 12 weeks of study. Over a period of 12 weeks, HbA1c decreased from 8.8% (1.4%) to 8.1% (1.6%) (P = 0.048) and weight decreased from 118.0 (19.7) kg to 107.4 (19.2) kg (P < 0.0001). Moreover, body mass index, waist and hip circumference, fasting blood glucose, triglycerides and high-density lipoprotein cholesterol improved significantly. After 1.5 years, insulin requirement and weight remained significantly lower than baseline. Participants who continued PRMR further reduced their HbA1c, weight and insulin dose. Two patients were able to stop insulin therapy altogether.
Conclusions: Energy-restricted PRMR was effective in reducing insulin requirement of type 2 diabetes patients with intensified insulin therapy accompanied by a reduction of HbA1c, weight and other cardiometabolic risk factors. With the continuous use of PRMR, glycaemic control might be improved in the long term.
Link to Study: Meal replacement reduces insulin requirement, HbA1c and weight long-term in type 2 diabetes patients with >100 U insulin/day
Download PDF: Meal replacement reduces insulin requirement, HbA1c and weight long-term in type 2 diabetes patients
Almased Clinical Studies: Preservation of Lean Muscle
Objective: To determine change of weight, body composition, metabolic and hormonal parameters induced by different intervention protocols.
Design: Randomized, controlled study including participants exhibiting a BMI between 27.5 and 35. Three different interventions containing lifestyle education (LE-G), or a substitutional diet containing a high-soy-protein low-fat diet with (SD/PA-G) or without (SD-G) a guided physical activity program.
Subjects: A total of 90 subjects (mean weight 89.9 kg; mean BMI 31.5), randomly assigned to one of three treatment groups.
Measurements: Change in body weight, fat mass and lean body mass measured with the Bod Pod device at baseline, 6 weeks and 6 months; change in metabolic and hormonal parameters.
Results: In all, 83 subjects completed the 6-months study. BMI dropped highly significantly in all groups (LE-G: -2.2+/-1.43 kg/m(2); SD-G: -3.1+/-1.29 kg/m(2); SD/PA-G: -3.0+/-1.29 kg/m(2)). Subjects in the SD-G and in the SD/PA-G lost more weight during the 6-months study (-8.9+/-3.9; -8.9+/-3.9 kg) than did those in the LE-G (-6.2+/-4.2 kg), and had a greater decrease in fat mass (-8.8+/-4.27; -9.4+/-4.54 kg) than those in the LE-G (-6.6+/-4.59 kg). In contrast, no significant intraindividual or between-group changes in the fat-free mass were seen. In all groups, metabolic parameters showed an improvement in glycemic control and lipid profile.
Conclusions: Our data suggest that a high-soy-protein and low-fat diet can improve the body composition in overweight and obese people, losing fat but preserving muscle mass.
Link to Study: Weight loss without losing muscle mass in pre-obese and obese subjects induced by a high-soy-protein diet
Download PDF: Weight loss without losing muscle mass in pre-obese and obese subjects
Abstract: The aim of the present study was to investigate the effect of a meal-replacement regimen vs. comprehensive lifestyle changes in overweight or obese subjects on intra-abdominal fat stores (Magnetic Resonance Imaging (MRI) measurements) and cardiometabolic risk factors. Forty-two obese men (n = 18) and women (n = 24) (age 49 ± 8 years; weight 96.3 ± 12.1 kg; BMI 32.7 ± 2.3 kg/m2) were selected for this randomized parallel-group design investigation. Subjects in the lifestyle group (LS-G; n = 22) received dietary counselling sessions and instructions how to increase physical activity. In the meal replacement group (MR-G; n = 20) meals were replaced by a low-calorie drink high in soy protein. After six months, subjects in the LS-G lost 8.88 ± 6.24 kg and subjects in the MR-G lost 7.1 ± 2.33 kg; p < 0.01 for changes within groups; no significant differences were found between the groups. Lean body mass remained constant in both intervention groups. MRI analyses showed that internal fat was significantly reduced in both groups to a comparable amount; the higher fat loss in the LS-G in the abdominal area was due to a higher reduction in subcutaneous fat. Both interventions significantly reduced components of the cardiometabolic risk profile and leptin levels. The decrease in the adipokines fetuin A and resistin was more pronounced in the MR-G. In conclusion, both interventions significantly reduced body weight, total fat mass and internal abdominal fat while preserving lean body mass. The reduction in the adipokines fetuin A and resistin was more pronounced in the meal replacement group suggesting an additional effect of soy protein components.
Download PDF: Internal Fat and Cardio metabolic Risk Factors Following a Meal-Replacement Regimen vs. Comprehensive Lifestyle Changes
Almased Clinical Studies: Cardiovascular Health
Background: As formula diets have demonstrated to be effective in reducing weight, we hypothesised that in patients with overweight or obesity and accompanied cardiovascular risk factors, combining a liquid formula diet with a lifestyle intervention is superior in reducing weight and improving cardiovascular risk factors than lifestyle intervention alone.
Methods: In this multicenter RCT 463 participants with overweight or obesity (BMI: 27-35 kg/m²; at least one additional co-morbidity of the metabolic syndrome) were randomised (1:2) into either a control group with lifestyle intervention only (CON, n = 155) or a lifestyle intervention group including, Almased, a liquid meal replacement (INT, n = 308). Both groups used telemonitoring devices (scales and pedometers), received information on healthy diet and were instructed to increase physical activity. Telemonitoring devices automatically transferred data into a personalised online portal and acquired data were discussed. INT obtained a liquid meal replacement substituting three meals/day (~1200 kcal) within the first week. During weeks 2-4, participants replaced two meals/day and during weeks 5-26 only one meal/day was substituted (1300-1500 kcal/day). Follow-up was conducted after 52 weeks. Intention-to-treat analyses were performed. Primary outcome was weight change. Secondary outcomes comprised changes in cardiometabolic risk factors including body composition and laboratory parameters.
Results: From the starting cohort 360 (78%, INT: n = 244; CON: n = 116) and 317 (68%, INT: n = 216; CON: n = 101) participants completed the 26-weeks intervention phase and the 52-weeks follow-up. The estimated treatment difference (ETD) between both groups was -3.2 kg [-4.0; -2.5] (P < 0.001) after 12 weeks and -1.8 kg [-2.8; -0.8] (P < 0.001) after 52 weeks.
Conclusions: A low-intensity lifestyle intervention combined with a liquid meal replacement is superior regarding weight reduction and improvement of cardiovascular risk factors than lifestyle intervention alone.
Download PDF: Almased meal replacement reduces more weight than lifestyle intervention alone
Background: Low-caloric formula diets can improve hemodynamic parameters of patients with type 2 diabetes. We, therefore, hypothesized that persons with overweight or obesity can benefit from a high-protein, low-glycemic but moderate-caloric formula diet.
Methods: This post-hoc analysis of the Almased Concept against Overweight and Obesity and Related Health Risk- (ACOORH) trial investigated the impact of a lifestyle intervention combined with a formula diet (INT, n = 308) compared to a control group with lifestyle intervention alone (CON, n = 155) on hemodynamic parameters (systolic and diastolic blood pressure (SBP, DBP), resting heart rate (HR), and pulse wave velocity (PWV)) in high-risk individuals with prehypertension or hypertension. INT replaced meals during the first 6 months (1 week: 3 meals/day; 2−4 weeks: 2 meals/day; 5−26 weeks: 1 meal/day). Study duration was 12 months.
Results: From the starting cohort, 304 (68.3%, INT: n = 216; CON: n = 101) participants had a complete dataset. Compared to CON, INT significantly reduced more SBP (−7.3 mmHg 95% CI [−9.2; −5.3] vs. −3.3 mmHg [−5.9; −0.8], p < 0.049) and DBP (−3.7 mmHg [−4.9; −2.5] vs. −1.4 mmHg [−3.1; 0.2], p < 0.028) after 12 months. Compared to CON, INT showed a pronounced reduction in resting HR and PWV after 6 months but both lost significance after 12 months. Changes in SBP, DBP, and PWV were significantly associated positively with changes in body weight and fat mass (all p < 0.05) and resting HR correlated positively with fasting insulin (p < 0.001) after 12 months.
Conclusions: Combining a lifestyle intervention with a high-protein and low-glycemic formula diet improves hemodynamic parameters to a greater extent than lifestyle intervention alone in high-risk individuals with overweight and obesity.
Download PDF: Almased Study - Improving Blood Pressure and Other Parameters
Almased Clinical Studies: Improved Quality of Life
Background: In addition to an increased risk for chronic illnesses, obese individuals suffer from social stigmatization and discrimination, and severely obese people may experience greater risk of impaired psychosocial and physical functioning. Lower health-related quality of life (HRQOL) has been reported among obese persons seeking intensive treatment for their disease. To aid in the treatment of obesity, meal replacements have been recommended as an effective therapeutic strategy for weight loss, particularly when consumed in the beginning of an intervention. Hence, the objective of this study was to assess the impact of two 12-month weight reduction interventions (one arm including a meal replacement) on changes in HRQOL among obese females.
Methods: This controlled trial compared two versions of a standardized 12-month weight reduction intervention: the weight-reduction lifestyle program without a meal replacement (LS) versus the same lifestyle program with the addition of a soy-based meal replacement product (LSMR). 380 women (LS: n = 190, LSMR: n = 190) were matched by age, gender, and weight (51.4 ± 7.0 yrs., 35.5 ± 3.03 kg/m2). This sample of women all completed the 12-month lifestyle intervention that was part of a larger study. The lifestyle intervention included instruction on exercise/sport, psychology, nutrition, and medicine in 18 theoretical and 40 practical units. Led by a sport physiologist, participants engaged in group-based exercise sessions once or twice a week. To evaluate HRQOL, all participants completed the SF-36 questionnaire pre- and post-intervention. Anthropometric, clinical, physical performance (ergometric stress tests), and self-reported leisure time physical activity (hours/day) data were collected.
Results: The LSMR sample showed lower baseline HRQOL scores compared to the LS sample in six of eight HRQOL dimensions, most significant in vitality and health perception (p < 0.01). After the intervention, body weight was reduced in both lifestyle intervention groups (LS: -6.6±6.6 vs. LSMR -7.6±7.9 kg), however, weight loss and HRQOL improvements were more pronounced in the LSMR sample (LSMR: seven of eight, LS: four of eight dimensions).
Conclusions: Our results show that HRQOL may improve among middle-aged obese females during a standardized lifestyle weight reduction program and may be enhanced by consuming a soy-based meal replacement product.
Download PDF: The impact of a weight reduction program with and without meal-replacement on health related quality of life
Abstract: While obesity impairs health-related quality of life (HRQOL), lifestyle interventions targeting weight reduction have been effective in improving HRQOL. Therefore, we hypothesised that a meal replacement-based lifestyle intervention, which has been shown to successfully reduce weight, would also improve HRQOL more effectively than a lifestyle intervention alone. In the international, multicenter, randomised-controlled ACOORH-trial (Almased-Concept-against- Overweight-and-Obesity-and-Related-Health-Risk), overweight or obese participants with elevated risk for metabolic syndrome (n = 463) were randomised into two groups. Both groups received telemonitoring devices and nutritional advice. The intervention group additionally used a protein-rich, low-glycaemic meal replacement for 6 months. HRQOL was estimated at baseline, after 3 and 12 months, using the SF-36 questionnaire, and all datasets providing HRQOL data (n = 263) were included in this predefined subanalysis. Stronger improvements in the physical component summary (PCS) were observed in the intervention compared to the control group, peaking after 3 months (estimated treatment difference 2.7 [1.2; 4.2]; p < 0.0001), but also in the long-term. Multiple regression analysis demonstrated that insulin levels and the achieved weight loss were associated with the mental component summary (MCS) after 12 months (p < 0.05). Thus, meal replacement-based lifestyle intervention is not only effective in weight reduction but, concomitantly, in enhancing HRQOL.
Download PDF: High-Protein, Low-Glycaemic Meal Replacement Improves Physical Health-Related Quality of Life
Almased Clinical Studies: Sports and Exercise
Objective: To determine changes in body composition, physical performance, metabolic and hormonal parameters induced by lifestyle counselling, resistance training and resistance training with soy protein based supplemention in middle aged males.
Design: Randomised controlled study consisting of resistance training without (RT-G) or with (RTS-G) a soy protein based supplement and a control group with lifestyle education only (LE-G).
Subjects: Forty healthy middle aged men (50–65 years, BMI 25–29.9 kg/m2).
Measurements: Changes in body weight (BW) and waist circumference (WC) were measured and body composition (BC), fat mass (FM), lean body mass (LBM) were measured by skin fold anthropometry at baseline and after 12 weeks of intervention. In addition, changes in physical fitness, metabolic and hormonal parameters (lipids, glucose, fructosamines, insulin, insulin-like growth factor-1, Leptin, human growth hormone, dehydroepiandrosterone, testosterone, hs-CRP, Il-6) were evaluated.
Results: Thirty-five participants completed the 12 week study. No significant changes in BW were noted although RM and WC dropped and LBM increased after training, particularly in the RTS group (FM 22.6 ± 5.5 kg to 21.2 ± 4.7 kg; LBM 68.5 ± 7.2 kg to 70.1 ± 7.4; p < 0.01). Subjects in the RTS group experienced more pronounced improvements in the strength measurements than the RT group. After the training intervention there were significant changes in hormonal and metabolic parameters as well as in glycemic control, particularly in the RTS group.
Conclusions: Our data suggest that resistance training, particularly in combination with a soy protein based supplement improves body composition and metabolic function in middle aged untrained and moderately overweight males.
Link to Study: Soy protein based supplementation supports metabolic effects of resistance training in previously untrained middle aged males
Download PDF: Soy protein based supplementation supports metabolic effects of resistance training
Introduction: Obesity is one of the greatest public health challenges of the 21st century. A combination of diet and exercise interventions have been shown to deliver stable weight reduction. M.O.B.I.L.I.S. is an interdisciplinary lifestyle modification program, aimed at lasting modification of exercise levels and diet with a view to an energy balanced lifestyle and healthy living skills.
Methods: Lifestyle changes are to be achieved via a 12-month exercise based intervention. This standardized training program is to be followed up at predetermined time intervals in a planned sample size of 4000 to 5000 obese adults (BMI 30 to 40 kg/m2). As target variables body weight, body mass index, and waist circumference were defined.
Results: One year results are now available for 32 groups and 454 obese participants. The results clearly demonstrate that lifestyle changes in obese individuals are possible and lead to an average weight reduction of 6.4 kg at 12 months.
Discussion: M.O.B.I.L.I.S. constitutes an effective, economic, and non-pharmacological therapy option for obese adults.
Link to Study: Exercise based lifestyle intervention in obese adults: results of the intervention study m.o.B.I.L.I.s - PubMed (nih.gov)
Download PDF: Exercise Based Lifestyle Intervention in Obese Adults
Abstract: The aim of this study was to determine the changes in endurance performance and metabolic, hormonal, and inflammatory markers induced by endurance stress (marathon race) in a combined strategy of training and dietary protein supplementation. The study was designed as a randomised controlled trial consisting of regular endurance training without and with a daily intake of a soy protein-based supplement over a three-month period in 2 × 15 (10 males and 5 females per group) endurance-trained adults. Body composition (body mass, BMI, and fat mass) was determined, and physical fitness was measured by treadmill ergometry at baseline and after 3 months of intervention; changes in exercise-induced stress and inflammatory markers (CK, myoglobin, interleukin-6, cortisol, and leukocytes) were also determined before and after a marathon competition; eating behaviour was documented before and after intervention by a three-day diet diary. Although no significant influence on endurance performance was observed, the protein supplementation regime reduced the exercise-induced muscle stress response. Furthermore, a protein intake of ≥20% of total energy intake led to a lower-level stress reaction after the marathon race. In conclusion, supplementary protein intake may influence exercise-induced muscle stress reactions by changing cellular metabolism and inflammatory pathways.
Link to Study: Continuous Protein Supplementation Reduces Acute Exercise-Induced Stress Markers in Athletes Performing Marathon
Download PDF: Continuous Protein Supplementation Reduces Acute Exercise-Induced Stress Markers in Athletes